Research. |
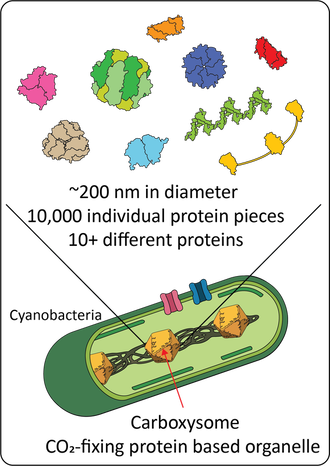
The cyanobacterial CO2 concentration mechanismRubisco, the primary CO2-fixing enzyme of the Calvin-Benson-Bassham cycle, is the most abundant protein on Earth. Despite this centrality, Rubisco is a relatively slow enzyme and fails to distinguish between CO2 and the competing off-target substrate of O2. To overcome these limitations bacteria, algae and plants have evolved different types of CO2 concentration mechanisms (CCMs). These systems create a locally high concentration of CO2 around Rubisco and thereby increase overall CO2 fixation rates. The cyanobacterial CCM consists of two major components: inorganic carbon transporters that actively accumulate bicarbonate in the cytosol and a protein organelle called the carboxysome.
Carboxysomes co-encapsulate the key enzymes for CO2 fixation, Rubisco and carbonic anhydrase, within a protein shell. These complex protein structures are built out of more than 10,000 distinct protein pieces that spontaneously self-assemble within the bacteria cell. We use a combination of biochemistry, enzymology, synthetic biology, structural biology, and bioinformatics to study various aspects (function, structure, assembly, regulation etc.) of the cyanobacterial CO2 concentrating mechanism. The acquired knowledge aims to lead the way for development of future microorganisms and crops with enhanced photosynthetic efficiencies. Enzymology and directed evolutionWe have a long interest in mechanistic enzymology and enzyme engineering. Our current work spans from fundamental understanding of carbonic anhydrases to directed evolution of biocatalysts. Currnet engineering project is part of a collaboration with Karin Stensjö aiming at using energy from the sun to produce biofuels in cyanobacteria.
|
Funding
|